2024 Year in Review
Restoring youthful function
2024 was NewLimit’s second full year of operations. Our key accomplishments:
>10X increase in the throughput of our Discovery Engine
Discovered TF sets that restore youthful gene expression while preserving cell type
Demonstrated that partial reprogramming with a drug-like molecule can restore youthful function in an animal model of liver disease
Along the way, we answered a series of open scientific questions that stood between us and reprogramming medicines. We believe that several of the risks present at our founding were overcome in the past 12 months.
Engine Cadence
NewLimit has built a set of technologies we refer to as our Discovery Engine to discover partial reprogramming payloads. Concretely, our Engine allows us to measure the effect of briefly activating a set of transcription factors (TFs) on the genes a cell is expressing and the functions it can perform.
In a single experiment, our Engine measures whether any of thousands of TF sets can make an old cell look younger – express genes closer to a young cell. For the most promising TF sets, we can then test if reprogramming makes old cells act younger – performing functions more like their youthful counterparts.
This year, we scaled the throughput of the Engine >10X along several axes. Our progress is best illustrated numerically:
The throughput of our Engine is the first-order input to the pace of our therapeutic discoveries. Outside NewLimit, we’re aware of <20 TF sets that have been tested for their effect on cell age. We now routinely run individual experiments that generate 50X as much data. Cumulatively, we’ve tested more than 500X as many TF sets as had been previously reported.
These screens resulted in our first promising discoveries. We found 100+ TF sets that restore youthful gene expression in old, human cells, and 5 sets that restore a youthful function.
Restoring youthful expression & preserving cell type
NewLimit was originally inspired by remarkable results from pluripotent reprogramming experiments. By activating just four TFs – the Yamanaka Factors – old cells can be reprogrammed back to a youthful state. These cells can give rise to a young animal with a normal lifespan. In old animals, transient activation of these factors can restore youthful gene expression and function across several tissues.
These discoveries are an existence proof that partial reprogramming can restore youthful function. Unfortunately, the Yamanaka Factors also suppress adult cell type programs, potentially turning adult cells into embryonic cells. This introduces a risk of neoplasia and limits efficacy, making it challenging to use them as medicines.
When we founded the company, we wondered if we could disentangle these two effects. Can we find sets of TFs that reprogram cell age, without reprogramming cell type? If so, medicines become tractable.
In 2024, we’ve found sets of transcription factors (TFs) that restore youthful gene expression, without abolishing cell type programs in both T cells (a key immune system cell) and hepatocytes (liver cells). These discoveries stand in contrast to the Yamanaka Factors which make old cells look younger at the expense of compromising their cell types.

Restoring youthful expression is compelling, but therapeutics may require these changes to be durable. A medicine that can be dosed every few weeks is more useful than one that must be dosed every day. We also discovered TF sets that restored youthful expression long after the TFs were turned off, mimicking durability after the withdrawal of a drug.
These results address two of the core scientific risks that were present at NewLimit’s founding. Youthful expression can be restored without abolishing cell type, and these effects can endure over time. There are many miles to go before we have medicines in hand, but these data suggest that nature is amenable to their existence.
Restoring youthful function with therapeutic molecules
Function is the final boss at NewLimit. We ultimately care most about making old cells act like young cells, restoring youthful functions that will treat aging & disease in patients. At the start of the year, we had reason to believe that we could build reprogramming medicines to achieve this goal, but no actual data.
In early winter, we developed the capabilities to produce lipid nanoparticle-mRNA formulations. These molecules are capable of briefly activating TF sets in hepatocytes in the liver. Importantly, they can also be turned into medicines. Pioneering LNP-RNA medicines are already approved in the US, including medicines that target hepatocytes in the liver and the COVID vaccines.
If partial reprogramming with these molecules can restore function, it offers a path to building reprogramming medicines.
We ran our first in vivo screens in human hepatocytes late in the autumn. To our surprise, even these early screens revealed multiple TF sets that restore youthful gene expression and function. Seeing functional improvements in a screen is encouraging, but we next wanted to confirm that these effects were robust.
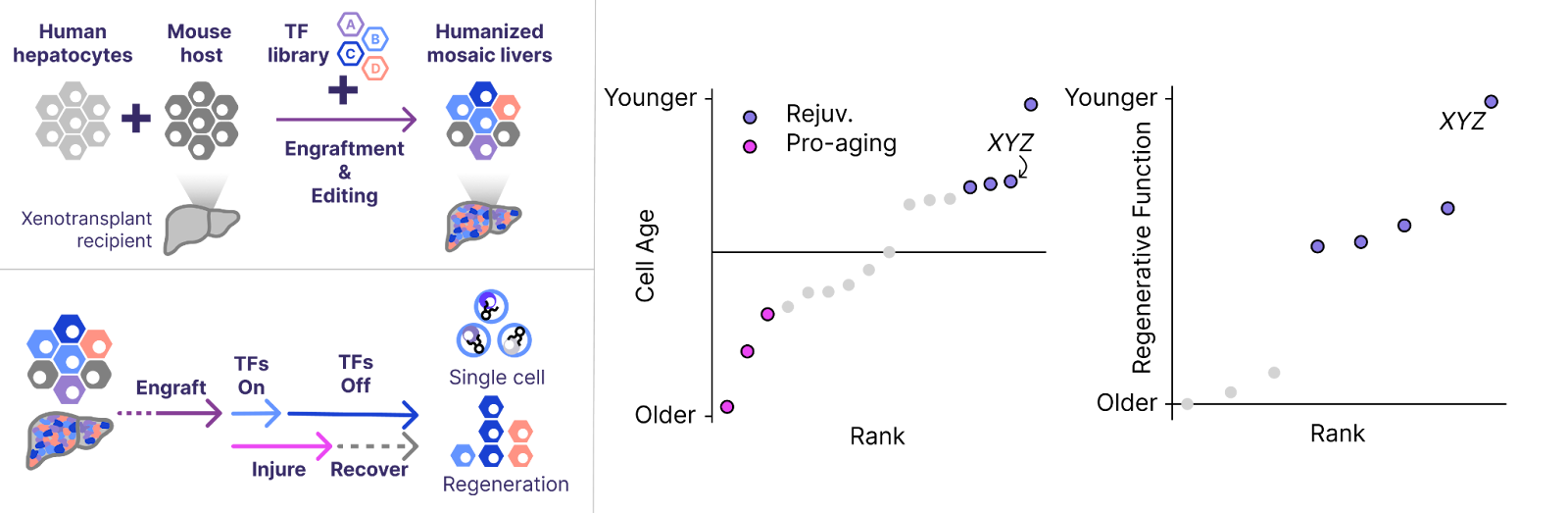
We formulated one of these TF sets as an LNP-mRNA and used it to treat a model of liver injury in old mice. Across several experiments, we found that partial reprogramming with these drug-like formulations was sufficient to restore youthful function in these old cells. The regenerative capacity that was lost with age remained latent and reemerged following reprogramming.
This model of age-related disease is only the first of many we plan to employ, but the results are nonetheless a promising sign that the medicines we envision are possible.

Technical innovations
Discoveries are downstream of the technologies that enable them. We’re especially proud of four technological innovations in our Engine last year.
In silico reprogramming performance scales as a function of data
Modern artificial intelligence systems frequently exhibit scaling phenomena – performance increases as a function of training data size, diversity, and compute.
We use in silico reprogramming (ISR) models to predict the effect of partial reprogramming with a given set of TFs, then prioritize our experiments using these predictions. These models are critical because the search space of TF sets is massive – 1016 combinations are possible! We can never search the entire space experimentally. In silico predictions allow us to target our experiments to only the most promising hypotheses.
This year, we showed for the first time that the performance of these predictions increases as a function of data scale. This is one of a handful of examples suggesting that scaling phenomena may exist in learned representations of life, in addition to the traditional artificial intelligence domains of computer vision and natural language.

We appear to still be in the linear regime of data scaling, suggesting that our models will continue to improve as we rev the Engine in 2025.
In vivo screening in human hepatocytes
In 2024, we initiated our Metabolism program focused on restoring youthful function in hepatocytes. One of the first challenges we encountered is that performing partial reprogramming screens at scale is challenging in this cell type.
Hepatocytes are not amenable to the in vitro culture techniques used in most screening systems. To overcome this challenge, we developed an in vivo screening technology for our program. This approach takes advantage of humanized liver mouse models to measure reprogramming effects in human cells inside a real, functioning liver.
To our knowledge, this is the first time a human hepatocyte screen has been executed in vivo. We believe the data emerging from this platform are more likely to reflect the biology of human aging & disease than more common animal model systems.

Functional assays of human cell age
Ultimately, NewLimit’s goal is to make old cells act young, not just look young. In order to achieve this goal, we first need to develop tools that can measure the functional defects that emerge in old cells.
We developed 11 new functional assays last year that discriminate between young and old cells, 5 in T cells and 6 in hepatocytes. While it may seem obvious that old cells are less functional than their young counterparts, measuring this rigorously is a surprisingly understudied area. We believe we now have the most robust assay suite for cell age that’s been developed for our therapeutic areas.
Combinatorial indexing for combinatorial screens
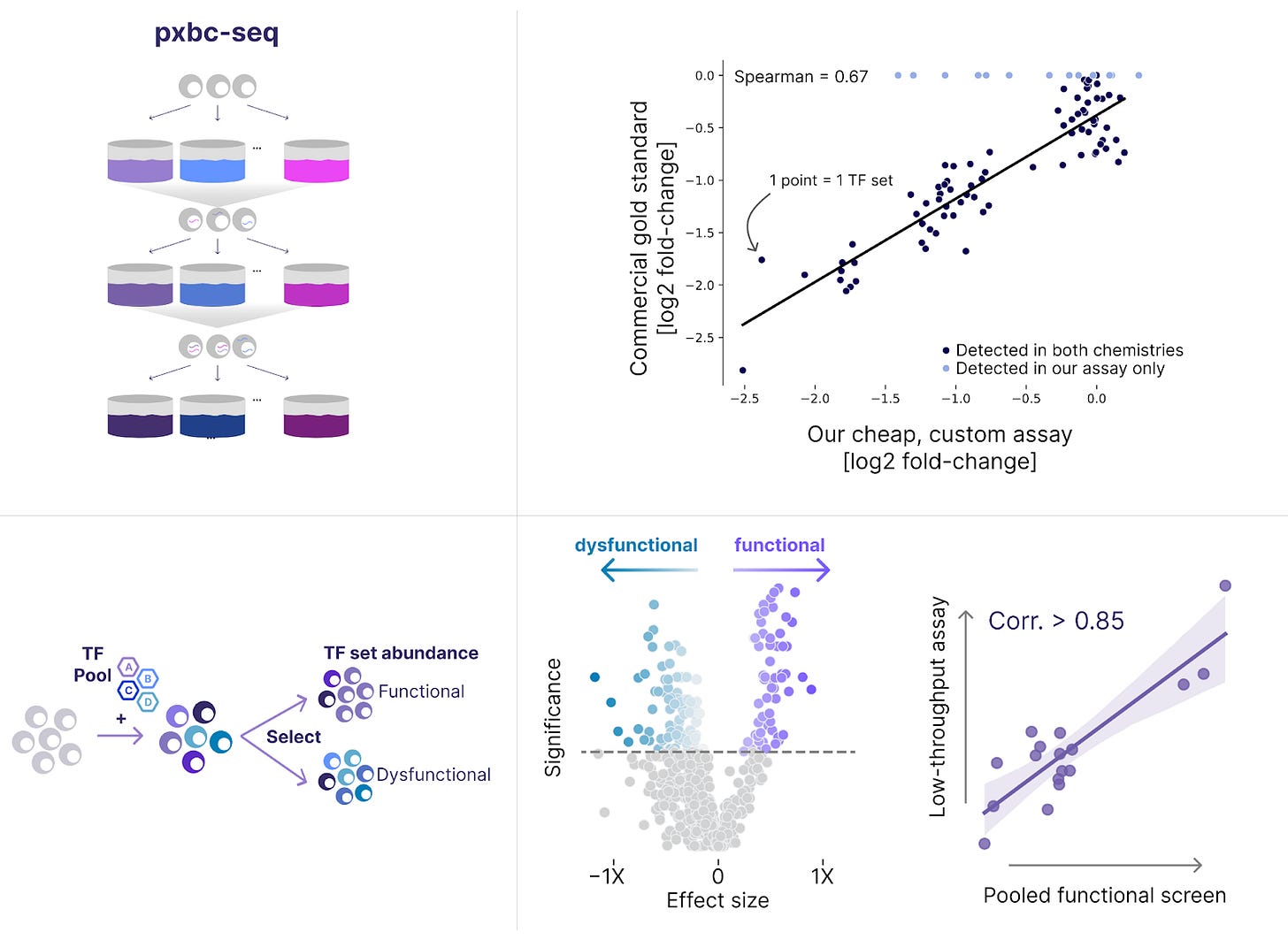
NewLimit is fairly unique in that we’re searching for therapeutic payloads that contain multiple genes. Screening for combinations introduces several technical challenges. One of them is quite simple – we need to be able to detect all of the TFs we’ve delivered to a given cell in order to measure their effect.
We developed an initial method to perform combinatorial TF screens in 2023. This year, we developed a new genomics method called pxbc-seq that builds upon combinatorial indexing methods to cut the cost of some of our screens by >100X. This technology allows us to perform selection-based screens cheaper than ever before. Already, we’ve deployed it to test >20X more TF sets in a functional assay than we could test with a traditional approach.
2025
The story of NewLimit’s past two years can be summarized concisely.
2023: We built the technology to discover partial reprogramming payloads in human cells.
2024: We discovered payloads that reprogram cell age while preserving cell type. We showed that reprogramming medicines are possible.
In 2025, we hope to progress some of these discoveries from research into the early stages of therapeutic development, laying the groundwork to bring medicines to patients.
There is a long road ahead before those medicines reach humans, but we’ve made more progress than we expected to date. We feel more confident today than ever that reprogramming medicines will have a marked impact on human healthspan.
Join us
The achievements we can claim thus far are a testament to the talent and effort of the team we’ve built. We continue to hire across multiple functions. If you’re excited by our mission, please reach out.


