2025 Year in Review
Entering our therapeutic epoch
We founded NewLimit in 2022 to develop reprogramming medicines that add happy, healthy years to people’s lives. We imagined it would take five years or more before our fundamental science was mature enough to start developing medicines.
The science moved faster than we anticipated. We began developing our first medicines ahead of schedule in 2025. Our key accomplishments:
Built our 1st preclinical candidate that restores multiple youthful functions in old hepatocytes
Developed frontier AI systems that increase our discoveries/$ by >2X
Discovered 30+ reprogramming payloads that restore youthful function in old cells
Launched our 3rd therapeutic program focused on Vascular biology & built multiple functional assays for cell age
Discovery cadence & metrics
NewLimit is built around a set of core technologies we call our Discovery Engine. The goal of our Engine is to discover transcription factor (TF) payloads that restore youthful function in old cells by reprogramming the epigenome, allowing us to treat disease and preserve health in old patients.
Our Engine first predicts which TF payloads will be effective with AI systems, then tests thousands of payloads at a time for their effect on old human cells. From these data, we can discover payloads that make old cells look young based on their active genes and act young based on their functional performance. We then build prototype medicines from the payloads we discover and test to see if we can treat or cure a disease in animal models of aging.
In 2025, we increased our Engine throughput many fold and decreased the cost of each new discovery. The numbers themselves communicate this most clearly.
Our team managed to increase both the scale and fidelity of our Engine this year. To our knowledge, we’ve now tested >1000X as many reprogramming payloads as the rest of the field combined. We tested more payloads in 2025 than in all of our cumulative experiments from 2022-2024. At the same time, we expanded our repertoire of gold-standard preclinical models and improved the fidelity of our scaled screens to discover functional hits, dramatically reducing the cost per discovery.
As a result of these improvements, we have now discovered 16 payloads that treat disease in gold-standard animal models, 36 payloads that restore youthful function in cells, 600+ that make old cells look young based on gene expression, and 0 → 1 preclinical candidate.
Entering early development
Therapeutic discovery transitions into development once a single molecular entity known as a preclinical candidate is chosen for in-depth evaluation. We imagined at the founding of NewLimit that it would take 5+ years to create one, but this year we delivered a candidate after only 3 years of operations.
Our bar to declare a preclinical candidate is higher than in many traditional settings. We will only advance prototype medicines to candidates if they are both safe and restore multiple youthful functions, suggesting that cell age is genuinely reversed by reprogramming . By contrast, many traditional therapeutic campaigns focus on a single target biology to rescue. Cell age is associated with health & disease progression in humans, so we hope that our more stringent criteria improve our chances of success in the clinic.
Our first candidate is based on a payload with pleiotropic activity, making old hepatocytes both look young and act young across multiple dimensions. We first discovered the payload in a humanized liver screen where it restored a youthful gene expression profile in old human hepatocytes and improved regenerative function. We then tested if this payload could rescue more than just this one function in this one context.
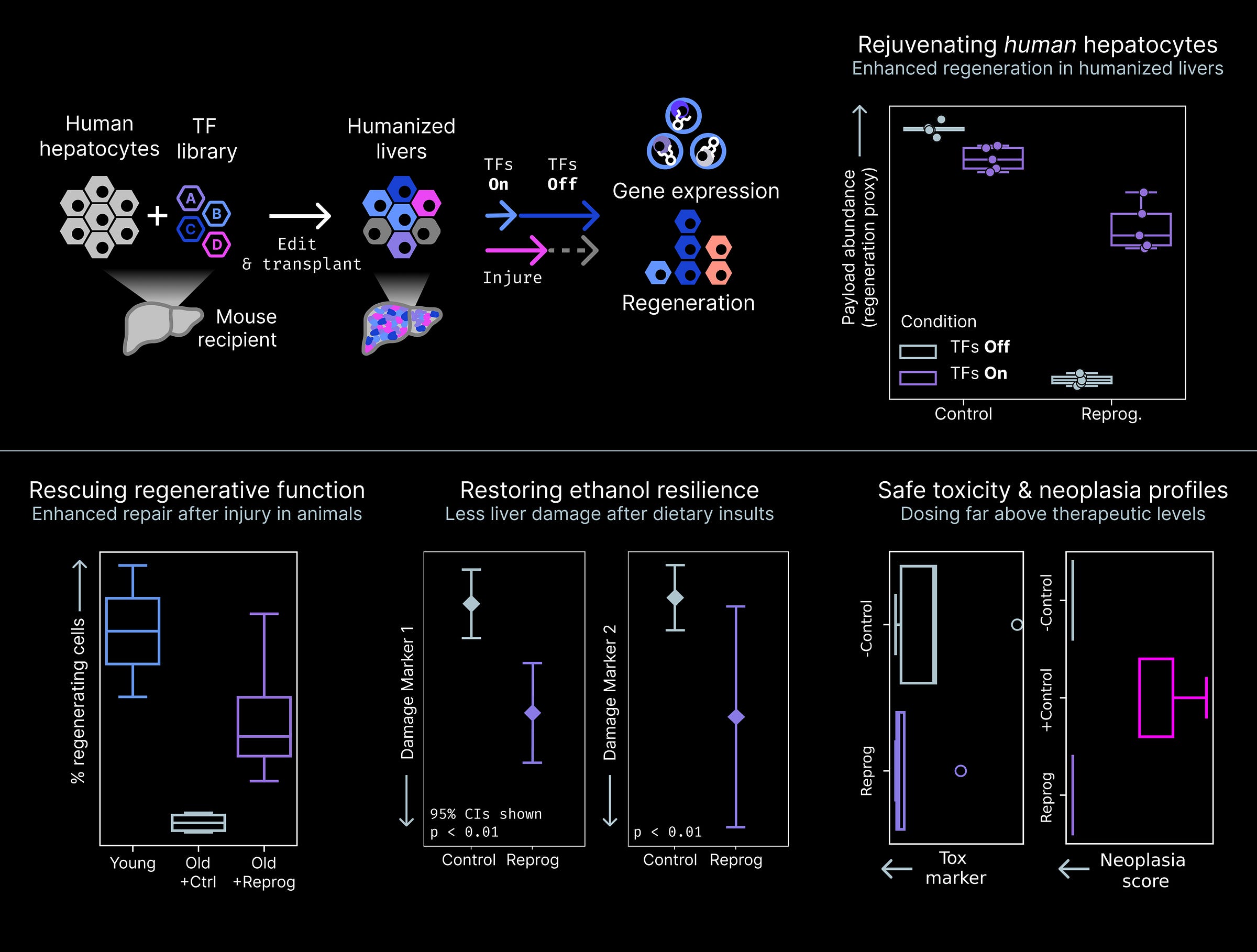
Old hepatocytes become more sensitive to damage and lose their ability to regenerate liver tissue after damage has occurred in animals. We found that our candidate payload not only restores resilience and prevents damage from alcohol, but also restores youthful regenerative capacity in a surgical injury model. The payload was safe in animals even at doses far exceeding the therapeutic level. Neither liver toxicity or neoplasia (e.g. tumors) were observed.
These rejuvenating effects convinced us that this payload warranted progression into development.
Our initial prototype LNP-RNA compositions for the candidate payload weren’t meaningfully optimized. We therefore executed a lead optimization campaign to improve both the potency and specificity of the payload prior to later stage studies. Our team established an RNA engineering process that yielded both a 1.6X increase in payload potency and an 8X increase in specificity for hepatocytes. These improvements are likely to yield a safer, more effective medicine.
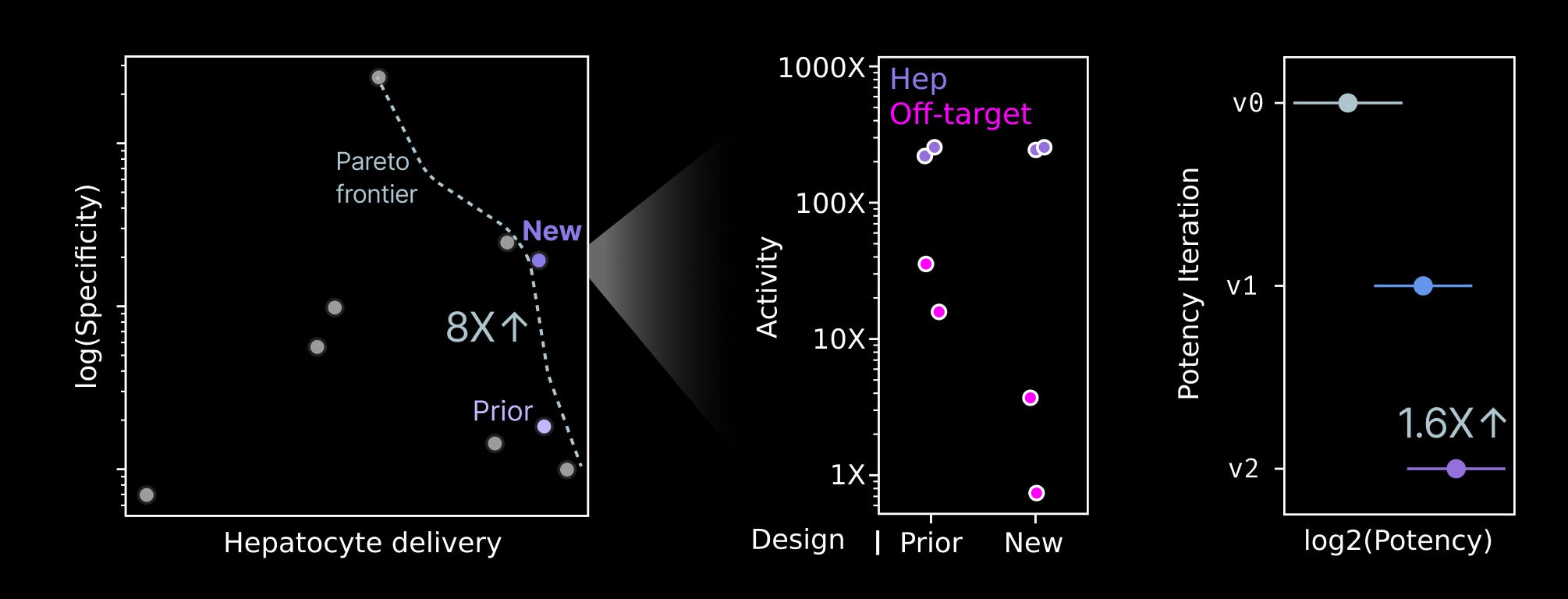
Creation of a preclinical candidate represents a meaningful milestone for NewLimit. More broadly, we believe it’s a milestone for the partial reprogramming field as one of the first therapeutic programs to reach this stage.
AI systems accelerate therapeutic discovery
Our reprogramming payloads are composed of combinations of transcription factors. There are >1016 possible combinations we might reasonably test, thousands of times more than the number of stars in the Milky Way galaxy. No matter how performant our experiments are in the world of atoms, we will never be able to exhaustively search this space. In order to discover an optimal reprogramming medicine, we need to effectively prioritize which hypotheses we test before ever entering the laboratory.
Only a few years ago, this problem was intractable. Human intuition is insufficient to reason through a hypothesis space of this scale. Modern artificial intelligence systems by contrast can incorporate a plurality of prior knowledge to design experiments that maximize our rate of discovery.
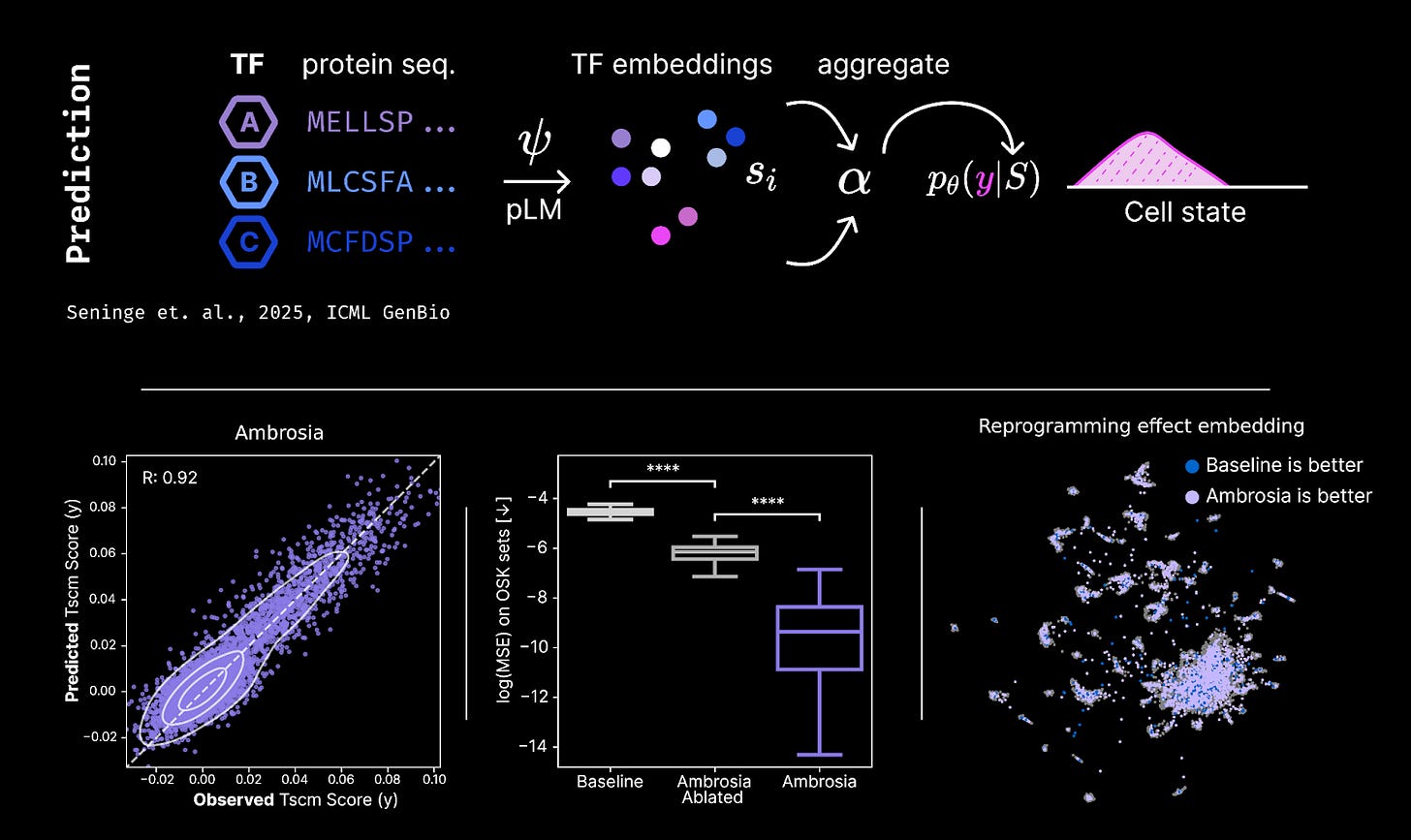
This year we introduced Ambrosia, the first AI system that can accelerate the design of reprogramming payloads. Ambrosia builds atop knowledge captured in foundational models of molecular biology and natural language, initializing our system with a strong prior derived from both human languages and nature’s languages. Our system first learns to predict the effect of arbitrary reprogramming payloads on both the state and function of old cells.
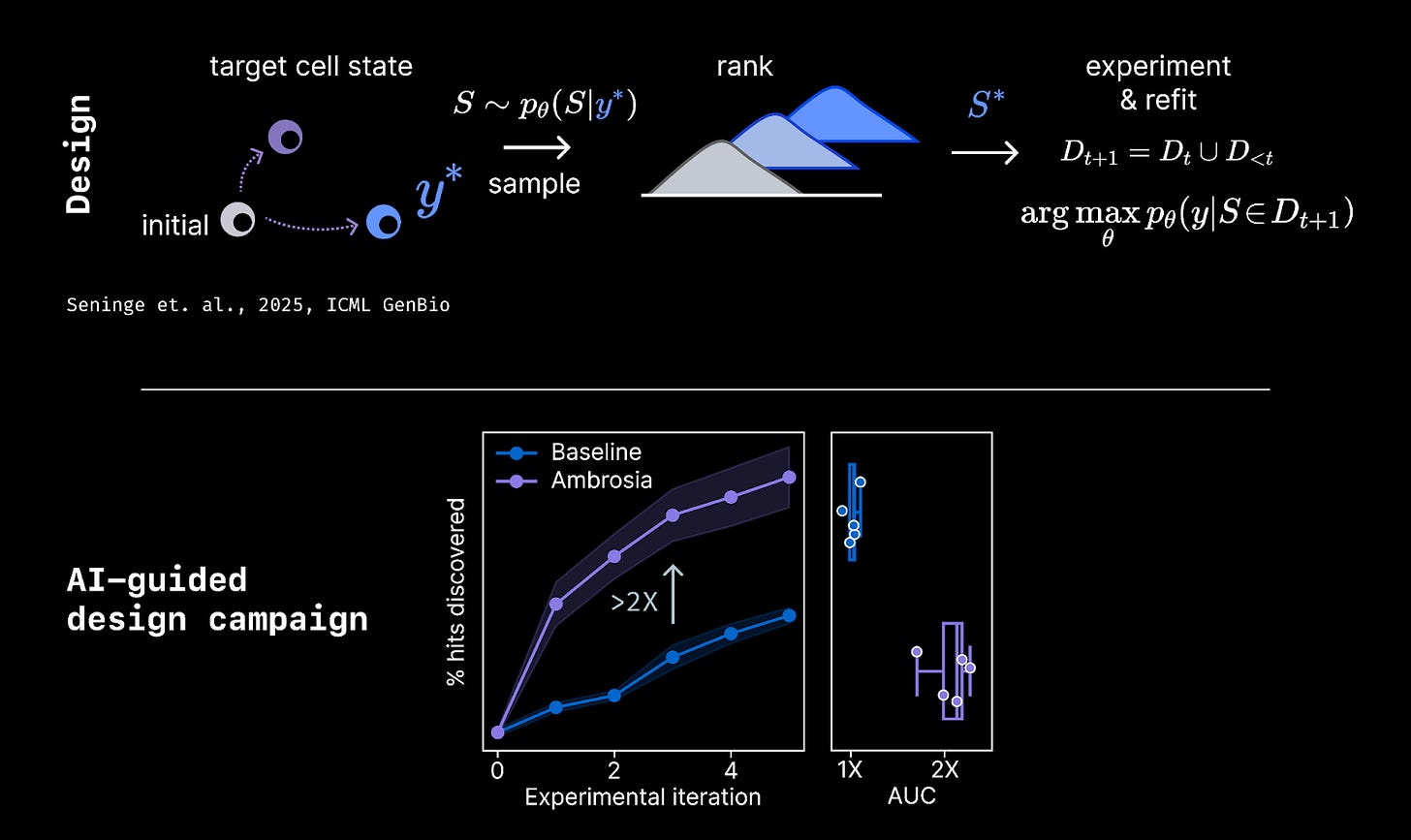
Given this discriminative model, Ambrosia can design reprogramming payloads that induce a target cell state or phenotype. The design process can learn continually from sequential experiments, allowing the system to iteratively navigate through the hypothesis space toward an optimal payload.
Performance on the initial prediction task is a special case of perturbation prediction (“virtual cell”) modeling, and Ambrosia achieved state-of-the-art performance relative to top baselines in the field. On the latter design task, our latest Ambrosia models improve our rate of discovery by >2X relative to baselines, directly increasing the number of discoveries we make per dollar invested. We believe this is among the first AI systems that has been integrated effectively into a large scale target discovery process.
The complexity of human pathology far exceeds the sophistication of most therapeutics. Our medicines have been constrained by our intelligence. It’s been intractable to design therapies that match biology’s complexity using our traditional systems of discovery. Reprogramming medicines are only now entering the realm of the possible due to the advent of AI systems that remove this intelligence constraint. If successful, the design of reprogramming medicines that extend human health will represent one of the more dramatic impacts of AI on our world.
Diversifying lineages
NewLimit’s ultimate ambition is to create reprogramming medicines to restore function across diverse cell types in the body. Our progress in hepatocytes and T cells this year convinced us to diversify our therapeutic bets to a third cell type.
In August, we launched a Vascular program focused on restoring function in endothelial cells, the cell type that lines blood vessels. Endothelial cells are present in almost all tissues, forming the transportation network that connects organs. Perhaps unsurprisingly, endothelial aging has detrimental effects across the body in the kidney, heart, lung, and potentially even the brain. By restoring function in the endothelium, we hope to provide near-term benefits in kidney disease and long-term benefits in many tissues.
Each of our therapeutic programs requires technology to (1) test many reprogramming payloads for their effect on cell age, (2) deliver reprogramming payloads as RNA to target cell types in vivo, and (3) measure the functional impact of reprogramming on cell age.
Our team demonstrated that our existing Engine technology transferred to endothelial cells with 0 modifications in just 3 months, 2X as fast as our prior record for onboarding a program. For delivery, we formulated a new lipid nanoparticle chemistry and achieved >60% delivery to kidney endothelial cells. These delivery tools unlock in vivo experiments, and our team also developed both in vitro and in vivo models of endothelial cell age in just four months.
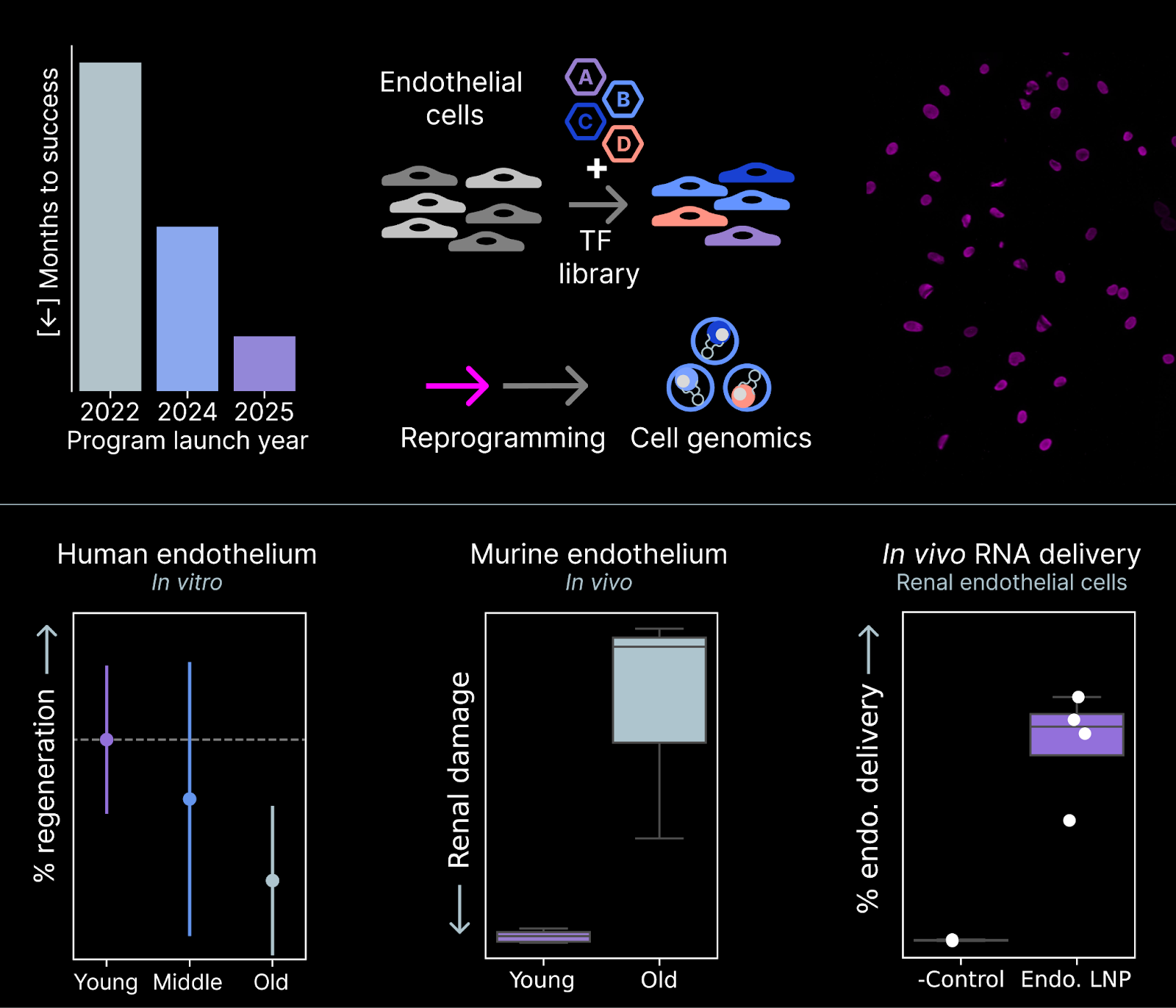
At the start of the year, our Vascular program was merely an idea. Today, we have assays to measure endothelial cell age, the screening technology to discover rejuvenating payloads, and the delivery tools to validate functional effects in vivo.
Molecular innovations
The scale of our discovery work in 2025 was enabled by a series of molecular inventions.
In vivo lymphocyte delivery: Measuring the effects of reprogramming in preclinical models requires an in vivo delivery chemistry to carry our prototype medicines to the right cell types. We implemented our first targeted LNP chemistry this year and achieved highly efficient delivery to T cells, approaching state-of-the-art performance. These tools have allowed us to test T cell reprogramming payloads in vivo for the first time.
Pooled molecular construction: Traditionally, our team relied upon a small number of arrayed molecular reactions to build reagents for our Discovery Engine screens. These steps require each hypothesis we test to exist for a time as a set of nucleic acids in an individual test tube. This year, our team developed a long-read sequencing method to eliminate these last few arrayed stages in our process, cutting the cost and turnaround time to build reagents for our screens by >5X.
Humanized liver engineering: NewLimit invented a method to run large-scale hepatocyte screens in vivo using a humanized liver system. Over the course of 2025, we made several improvements to this system and achieved a >10X increase in the throughput/$.
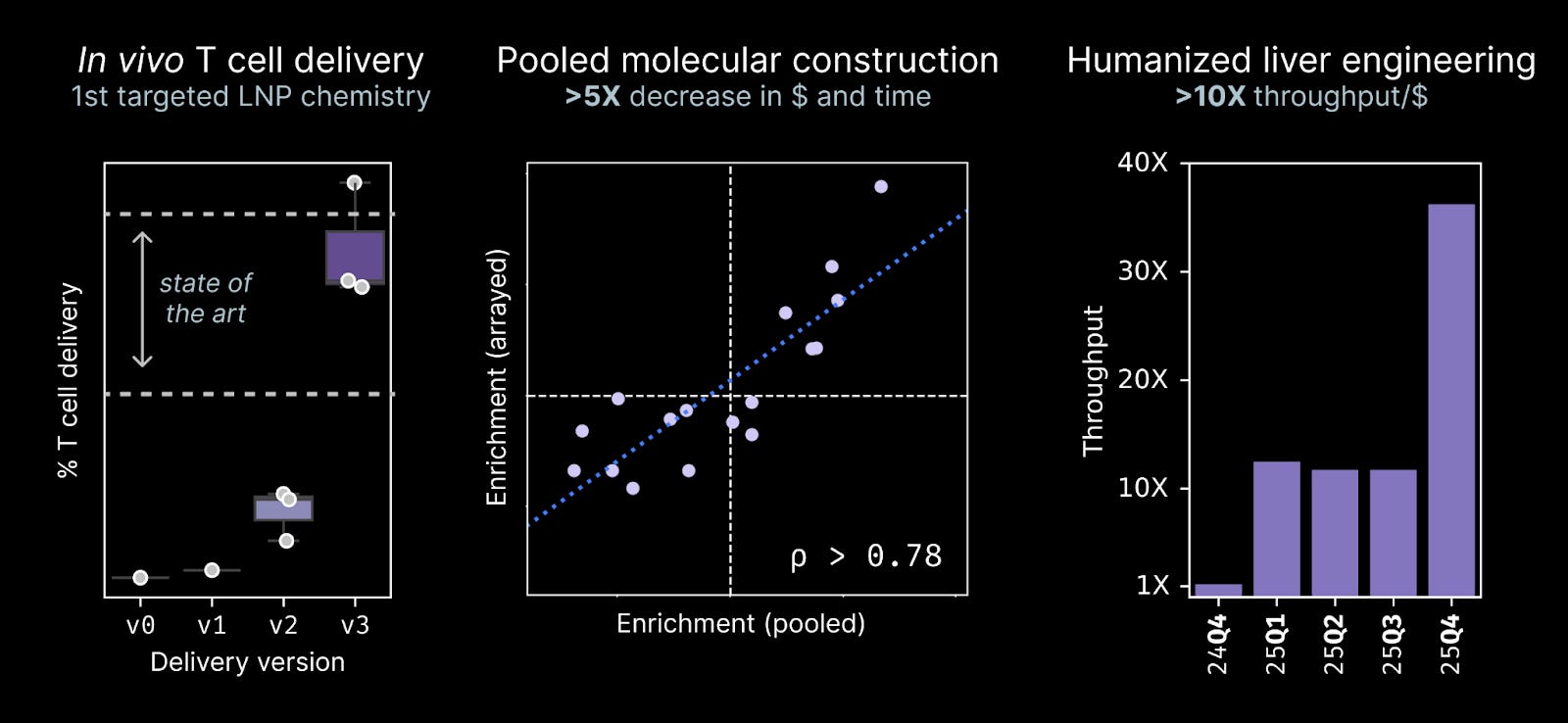
No one single molecular advance underlies our Discovery Engine technology. Rather, a series of these technical improvements have compounded over the course of three years to yield a reproducible system for discovering and validating new reprogramming medicines.
2026
NewLimit’s first two years were focused on a set of fundamental research and technology problems. We built a system to search for reprogramming medicines, discovered the first payloads that can reprogram cell age, and demonstrated that restoring youthful function in old cells was possible. In 2025, we created the first medicine we hope to bring to human trials and showed that it can rescue multiple youthful functions in old cells.
2026 will be the year that we transition from purely a research enterprise to an integrated research & development organization. Effective execution in this next stage will allow us to bring reprogramming technology into human patients. If these medicines are successful, we believe they are among the most valuable products possible.
Join NewLimit
Our progress to date is the product of a talented team of scientists, engineers, & operators. We’re recruiting across the company as we enter our next stage of growth. Please reach out if you’re interested in joining us.

