July // August 2025 Progress Update
Annexing new therapeutic territory
We’ve spent the latter months of summer moving our most advanced prototype medicines from discovery toward development. Our most recent data have convinced us that we’re on track to launch a human trial in the coming years, and these are the earliest steps in the process. Alongside this work, we’ve deployed new screening technologies and launched a new therapeutic program.
A few highlights:
+2 prototype medicines with efficacy in preclinical models of liver disease
+14 transcription factor sets that restore youthful function in T cells
+8 transcription factor sets that restore youthful function in hepatocytes
>4000 transcription factor sets tested across therapeutic areas
1.12X improvement in real discovery rates from reprogramming AI
0 → 1 lead payloads with translational chemistry
0 → 1 screens in endothelial cells
People
We’ve welcomed several new members to the NewLimit team.
Greg Farber joined our Vascular team as a Scientist. Greg previously worked in the Regenerative Medicine team at Genentech, completed a postdoctoral fellowship with Li Qian at the University of North Carolina, and earned a PhD at Cornell.
Quinn Lyon joined our Immunology team as a Senior Research Associate. Quinn previously worked at Deciduous Therapeutics.
Robin Meyers joined our Predictive Modeling team as a Data Scientist. Robin previously completed a PhD at Stanford with Paul Khaveri exploring the epigenetic regulation of epithelial fate decisions.
Daniella Rastelli joined our Metabolism team as a Senior Research Associate. Daniella previously worked on metabolic disease models at Takeda.
Meghan Sedovy joined our Vascular team as a Senior Research Associate. Meghan previously completed a PhD at Virginia Tech.
Benjamin Humphreys and Matthew Breyer have joined our Scientific Advisory Board to support our new Vascular program.
Constructing therapeutic molecules
NewLimit has been building prototype RNA medicines in our Metabolism program for nearly a year. Our earliest prototypes used lipid and RNA molecules that are effective for work in animal models, but preparing for human trials requires an even higher quality and performance standard.
We spent the past few months upgrading our early prototypes to use clinical-grade lipid chemistries. We initially encountered performance challenges when we simply swapped in the new chemistry for the old without iterating on our manufacturing methods. Our team took these challenges in stride and performed an optimization campaign that eventually achieved superior delivery efficiency to our prior prototypes.
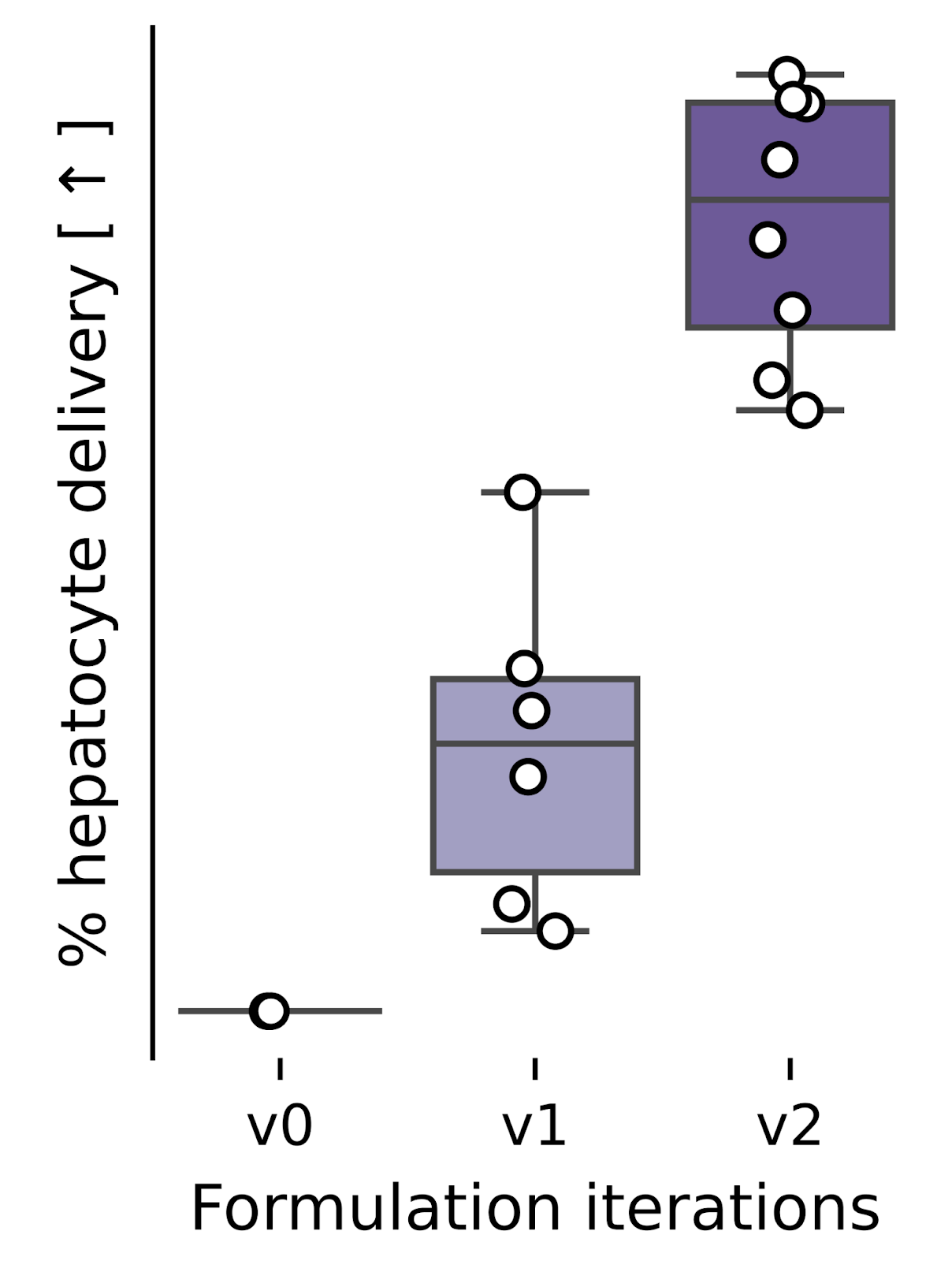
This new chemistry worked so well in fact that we’ve switched to using it for all of our future work in the program. Over the coming months, we’ll similarly be engineering optimal RNA sequences for subsequent use in humans.
Inventing medicines is a game of serial prediction, a parlay bet on the outcome of a product once it reaches a human trial. The more similar a preclinical system is to the eventual medicine, the more likely those early results are to replicate in human patients. While difficult to implement at the earliest stages, we believe our investments in deploying clinical-grade chemistry early in our discovery process increase our chances of delivering products that provide real benefit for humans.
Selecting for resilience
NewLimit’s Discovery Engine screens measure both phenotypes and functions of old cells treated with reprogramming interventions. Our phenotypic screens ask whether reprogramming makes an old cell look like a young cell, whereas our functional screens ask whether reprogramming makes an old cell act like a young cell. For our Metabolism program focused on restoring hepatocyte function, we’ve historically measured the regenerative capacity of human hepatocytes in a humanized liver as our main functional read-out.
Last month, we introduced a second functional screening paradigm to measure the resilience of hepatocytes to injury from toxic diets. In this setting, we engineer each hepatocyte in the liver to harbor a different combination of transcription factors. We then reprogram the cells with a dose mimicking an RNA therapy, then challenge the liver with an alcohol diet.
Reprogramming payloads that make hepatocytes more resilient to alcohol toxicity become more abundant in the liver over time as cells with other payloads die off. The system allows us to directly measure how well reprogramming protects hepatocytes from damage in hundreds to thousands of payloads at a time.
To evaluate the performance of this system, we retested several reprogramming payloads that increase or decrease resilience in gold-standard preclinical models. We know the effects these payloads should have, so they serve as a set of positive controls that allow us to measure the success of the screen. We were excited to find that our positive controls were strongly enriched for their expected effects, arguing that the screening system itself is performant.
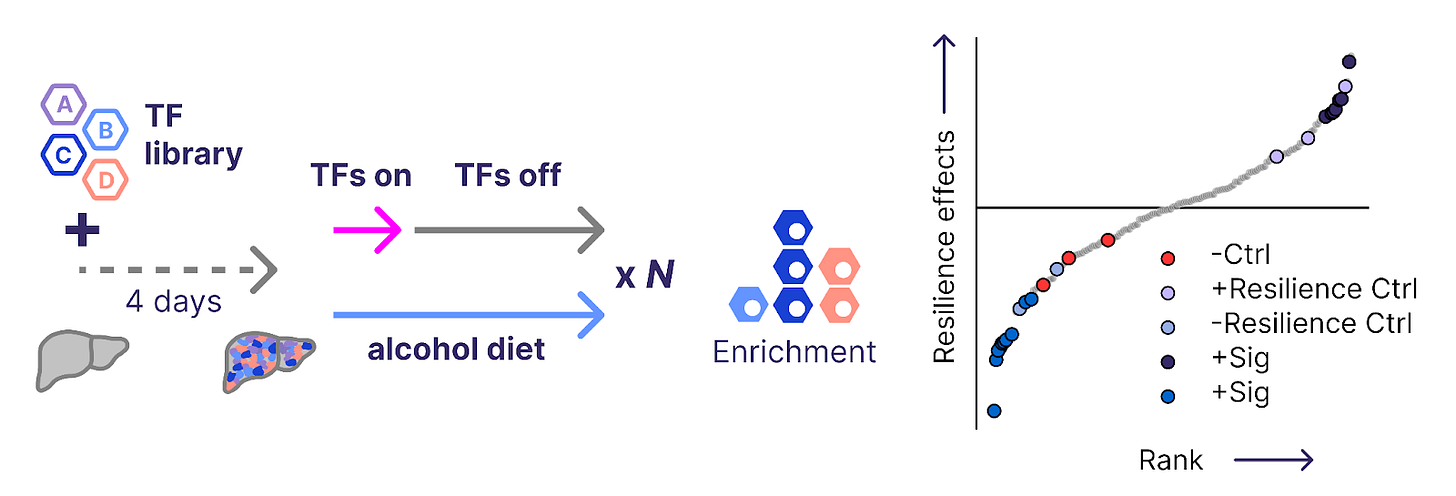
Already, these first screens have revealed new therapeutic payloads with efficacy in animal models. We look forward to the many discoveries to come.
Restoring function in the body’s transit system
In principle, we believe epigenetic reprogramming can restore youthful function in the majority of cell types within the human body. We’ve intentionally narrowed our focus to a small number that maximize the potential impact of these medicines in NewLimit’s early years. With the rapid progress of our lead program in hepatic metabolism, we believe it’s now time for us to expand the palette of cell types we study. We launched our third therapeutic program focused on restoring function in endothelial cells of the vasculature as a result.
Why endothelial cells?
Endothelial cells line the veins, arteries, and capillaries that allow blood to circulate to all the other tissues in your body. They are one of the few cell types that participates in the health and function of every other tissue. As we age, our endothelium atrophies and becomes pathologically permeable. Our aged tissues have inadequate access to the circulation and are exposed to undesirable signals that promote disease. Endothelial dysfunction contributes to aging pathology in the renal, cardiovascular, and cognitive systems among others. Restoring youthful function in endothelial cells may therefore offer diverse benefits.
Our Vascular team is initially focused on improving function in aged & diseased kidneys. There’s strong evidence that kidney endothelial cells lose function with age, and that restoring this function can provide benefit to patients with otherwise intractable and debilitating disease. Kidney function declines in most older patients, so we imagine that a medicine restoring youthful endothelial function could one day benefit nearly everyone over a certain age.
Each of our therapeutic programs begins by translating our Discovery Engine technology to the new cell type of interest. Here, we found that our existing Engine tools were performant in endothelial cells with zero modifications, demonstrating the generality of our approach.
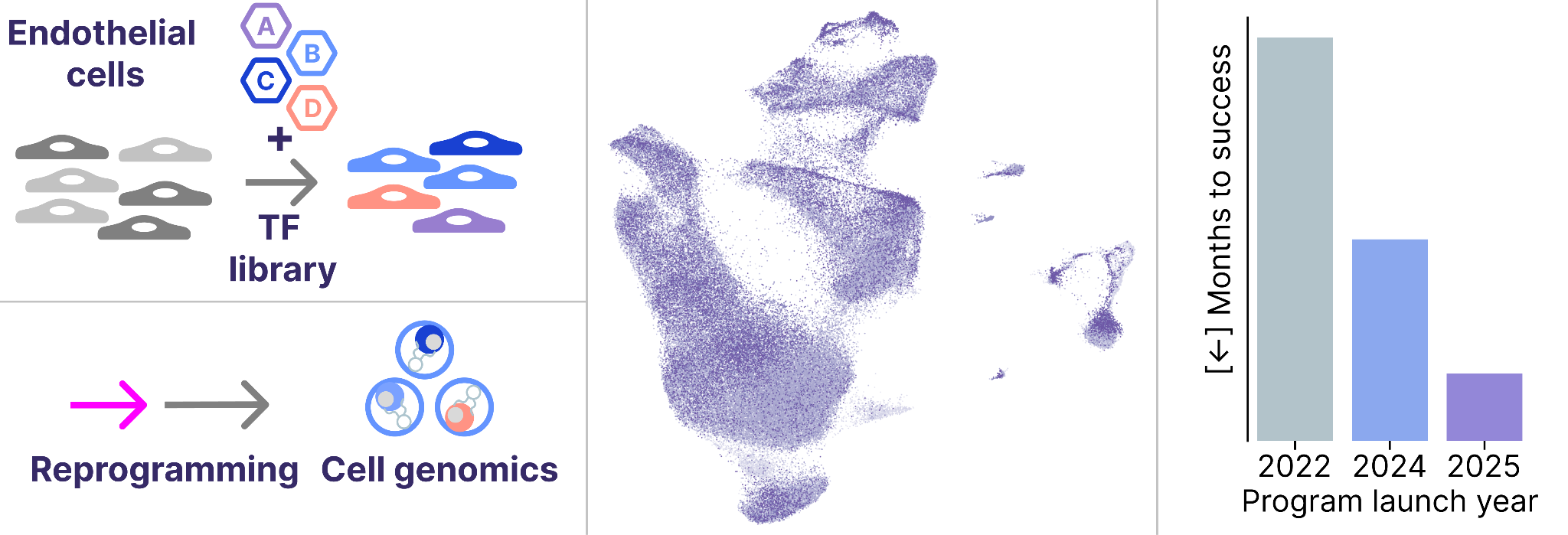
As a result, we completed the first successful screen for our Vascular program just weeks after launching the program. We’re now heads down building functional assays & preclinical models to measure the performance of payloads we discover in these screens.
In silico reprogramming with real world feedback
Modern artificial intelligence systems are often trained using feedback from humans or verifiable rewards to improve the quality of generated samples. We face an analogous challenge for our in silico reprogramming models. Our models design a number of reprogramming payloads intended to achieve a desired cell state (e.g. young, but keeping the same cell type). To determine if these designs are effective, we need to verify their performance in the real-world through laboratory experimentation.
We recently executed the first large scale reprogramming screen with a new “epistatic” variant of our in silico reprogramming models. These models take as input a representation of the transcription factor genes we deliver to aged cells and predict as an output the expected effect on cell age and identity. We can then use these models to design experiments that test payloads most likely to achieve our desired cell states. If our models are effective, they should allow for us to make more discoveries per experiment by prioritizing the payloads to test more effectively than human alternatives.
Our improved models demonstrated just this result. A large screening experiment designed with these models yielded 12% more discoveries than our previous state-of-the-art model. This represents a rare case where improvements in the rapidly refined world of bits lead to more value in the high latency world of atoms. We hope to share more on the technical details behind the iteration soon.
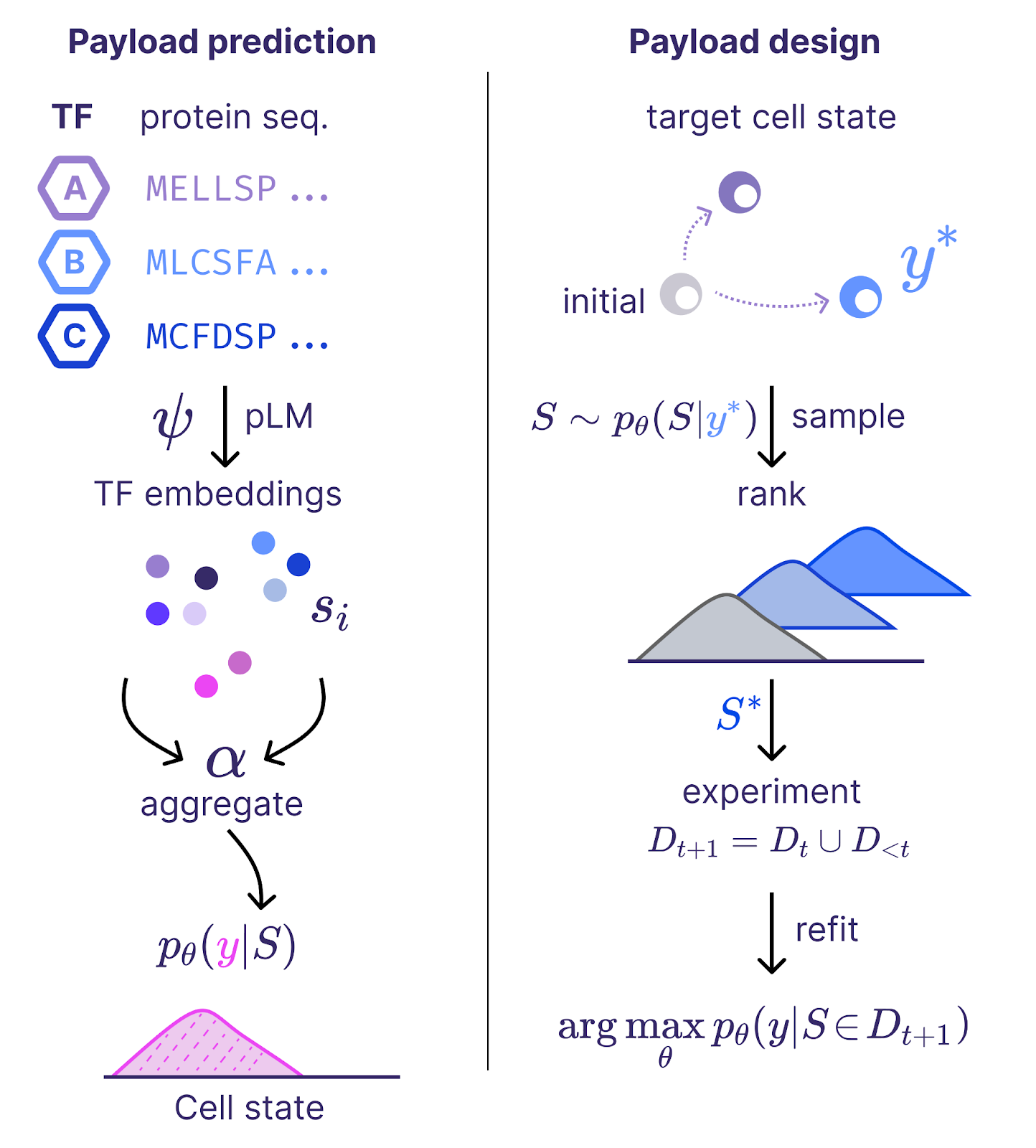
These new experimental data allow us to build even more performant models, following the data scaling laws we’ve reported previously. We shared technical details behind an earlier version of our in silico reprogramming approach at ICML in July – check out the paper!
Elsewhere
We had the pleasure of talking about NewLimit in a few other venues over the past couple of months. Check out these conversations at the links below.
Dwarkesh Podcast, Evolution designed us to die fast; why we can change that
ICML 2025 GenBio, In silico design of epigenetic reprogramming payloads
The Story Company, The Secret to Accelerating Longevity
Work with us
There are a finite number of problems where the outcome matters on the timescale of a century. Creating abundant intelligence, unlocking sources of energy too cheap to meter, and allowing humanity to explore the stars may be a few examples. We believe that expanding the number of healthy, happy years in each human life is one of these few, rare problems with enduring impact.
Making progress toward our mission is commensurately difficult, but offers the opportunity to perform the best work of a career. If you’re compelled to pursue goals with deep meaning and excel to the top of your craft, please consider joining our team.


