September // October 2025 Progress Update
Sequential optimization
We are close to holding our first clinic-bound reprogramming medicine in hand. Scientists across each of our teams have spent the past several months building the final version of a therapeutic payload that restores youthful function in hepatocytes. We plan to move this medicine into human clinical studies in a few years. Along with our efforts to build this therapeutic, we also discovered promising new payloads and built enabling tools for our follow on programs.
A few numerical highlights:
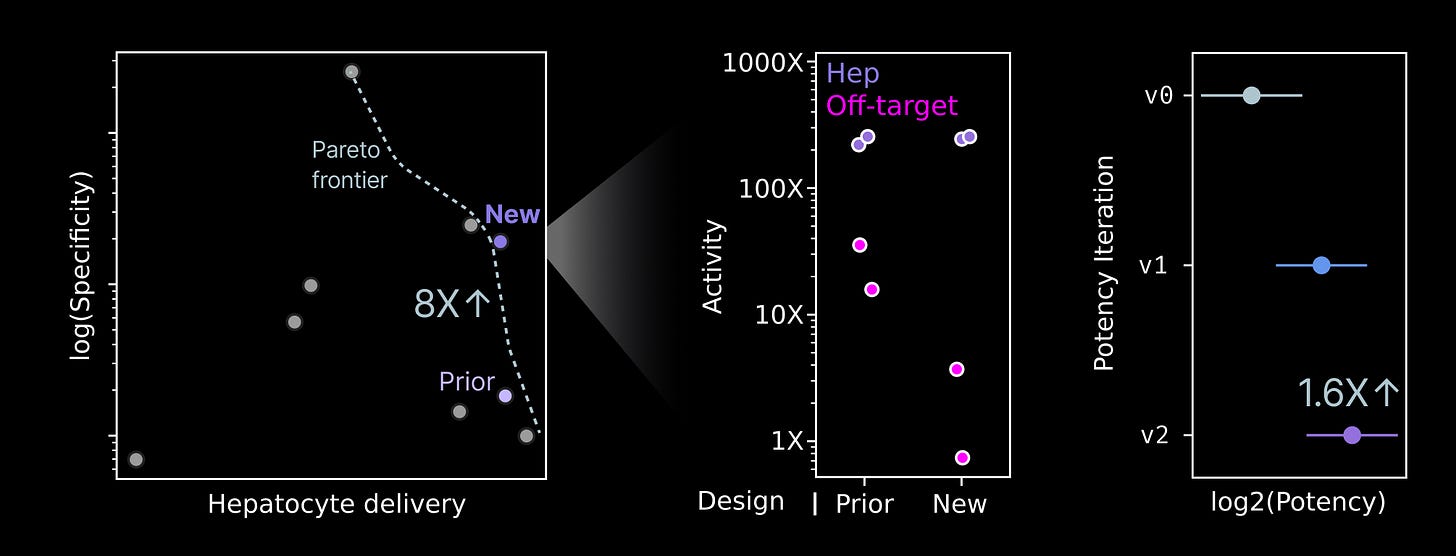
8X the specificity & 1.6X the potency of our lead prototype medicine
+2 TF sets with therapeutic benefit in liver disease models
0 → 1 functional assays for endothelial cell age
>20 TF sets that restore youthful phenotypes in old hepatocytes
>3000 TF sets tested across programs
5X decrease in molecular construction costs
People
These results reflect the contributions of a focused team. We’ve been excited to welcome several talented scientists recently.
Angela Boroughs joined as a Director to lead our Immunology therapeutic program. Angela previously led synthetic biology & immunology teams at ArsenalBio and directed the development of a clinical stage CAR-T cell therapy. She holds a Ph.D. in Immunology from Harvard University.
Daniel Baca joined as a Research Associate on our Single Cell Technology team from ArsenalBio.
Sita Chandrasekaran joined as a Scientist on our Epigenetic Editing team. She previously completed Ph.D. training with Patrick Hsu and postdoctoral work with Silvana Konnermann at the Arc Institute.
Sean Hsieh joined as a Scientist on our Metabolism team. He previously completed a Ph.D. with Hao Zhu at the University of Texas Southwestern.
Engineering therapeutic molecules
Over the course of 2025, NewLimit has identified many reprogramming payloads that restore youthful function in old hepatocytes. In September, we selected one of these lead payloads to progress into later stages of therapeutic development.
This selection triggered the start of a lead optimization campaign. We set out to design mRNA sequences encoding our payload that (1) maximize the potency of the prototype medicine and (2) improve the selectivity of the medicine for activity in hepatocytes.
Sequences that translate from mRNA into protein more efficiently are expected to have a higher potency per unit dose. We implemented a range of sequence optimizations and evaluated their translational efficiency across a battery of different human cell types. We ultimately designed a sequence that increased the potency of our payload by 1.6X relative to our starting sequence.
mRNAs are not translated into proteins equally across cell types. Within the cytosol of the cell, a range of RNA binding proteins, RNA-guided nucleases, and the abundance of translational machinery dictate how many proteins are translated from each mRNA molecule. One cell type might make hundreds of proteins from an mRNA sequence, whereas another cell type might produce only one. We can take advantage of these differences to build a medicine that has minimal activity outside the cell type of interest, improving the safety profile.
Similar to our potency optimizations, we designed a range of sequences and eventually achieved an 8X improvement in the specificity of our prototype medicine for hepatocytes relative to off-target cell types. Combined with other optimizations, our prototype now achieves a dramatic specificity for hepatocyte activity relative to off-target cell types.
We’re now evaluating performance of the fully optimized product across a range of preclinical models and hope to progress toward formal development soon.
Restoring youthful resilience
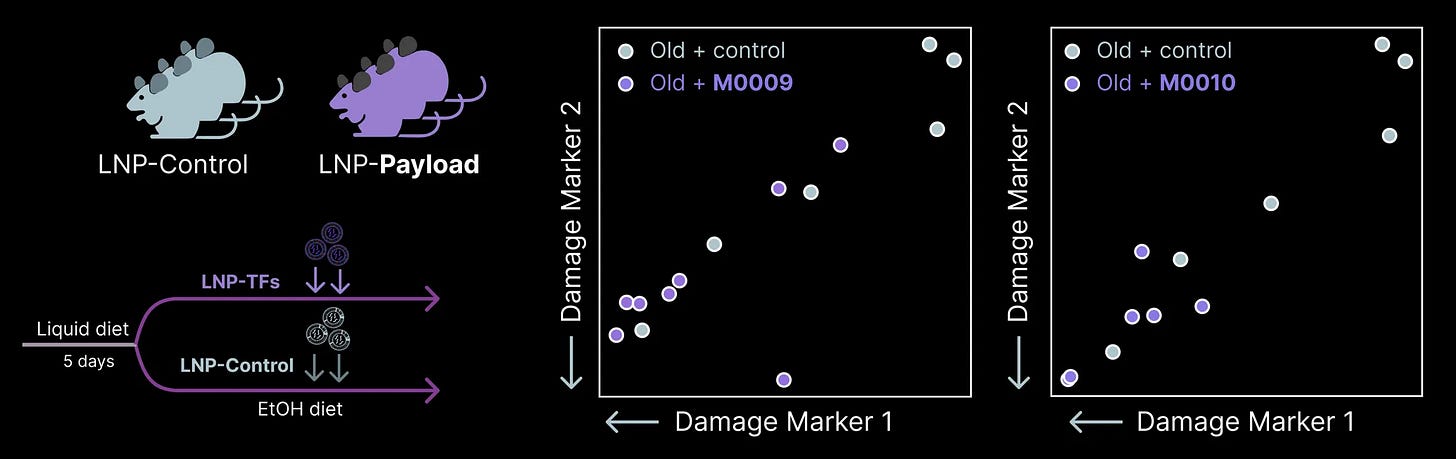
Alongside the progression of our first assets, we continue to discover new reprogramming payloads through our Discovery Engine. In October, we validated the performance of +2 new payloads that restore youthful function, making old cells act young.
Each of these payloads originally emerged from our in vivo screens in hepatocytes. Neither has been extensively explored in prior work to our knowledge.
After discovering each payload in a screen, we formulated prototype medicines and tested their ability to protect old hepatocytes from a diet-induced injury. We then measured the amount of damage to the liver using circulating biomarkers of damage. These biomarker proteins are only produced inside hepatocytes. If we find them in the blood, it suggests hepatocytes have recently experienced damage and “leaked” these proteins into the blood. The less we detect, the healthier the liver is.
Both of these new payloads restored a youthful level of resilience to a dietary insult, dramatically lowering the amount of these damage markers we detected in the blood.
We’ve continued to discover new payloads like this month after month. These results give us confidence that our Engine can produce more and more discoveries over time.
In vivo reprogramming of T cells
Hepatic reprogramming is only one of several therapeutic applications we’re pursuing. We’ve previously discovered a range of reprogramming payloads that restore youthful cytotoxic capacity and stem-like states to old human T cells. The next stage in the development of these payloads is to test whether they can rescue function in a more complex animal model. Before we can test their effects directly, we need to build molecular tools to deliver reprogramming payloads to T cells in vivo.
Lipid nanoparticles (LNPs) are the current state-of-the-art method for delivering RNA payloads to cells in the body. They can be readily delivered to hepatocytes in the liver because hepatocytes naturally pull lipids (”fats”) from the bloodstream as part of their energy production and storage function. Delivering to other cell types requires more nuance. Typically, a targeting molecule is introduced on the outside of the lipid nanoparticle to guide it to specific cell types.
We’ve worked through a series of iterations to arrive at a targeted LNP chemistry that achieves delivery to T cells in mice. Our initial attempts barely achieved delivery of RNA to T cells, but over the course of a few months we were able to nearly match the current state-of-the-art.
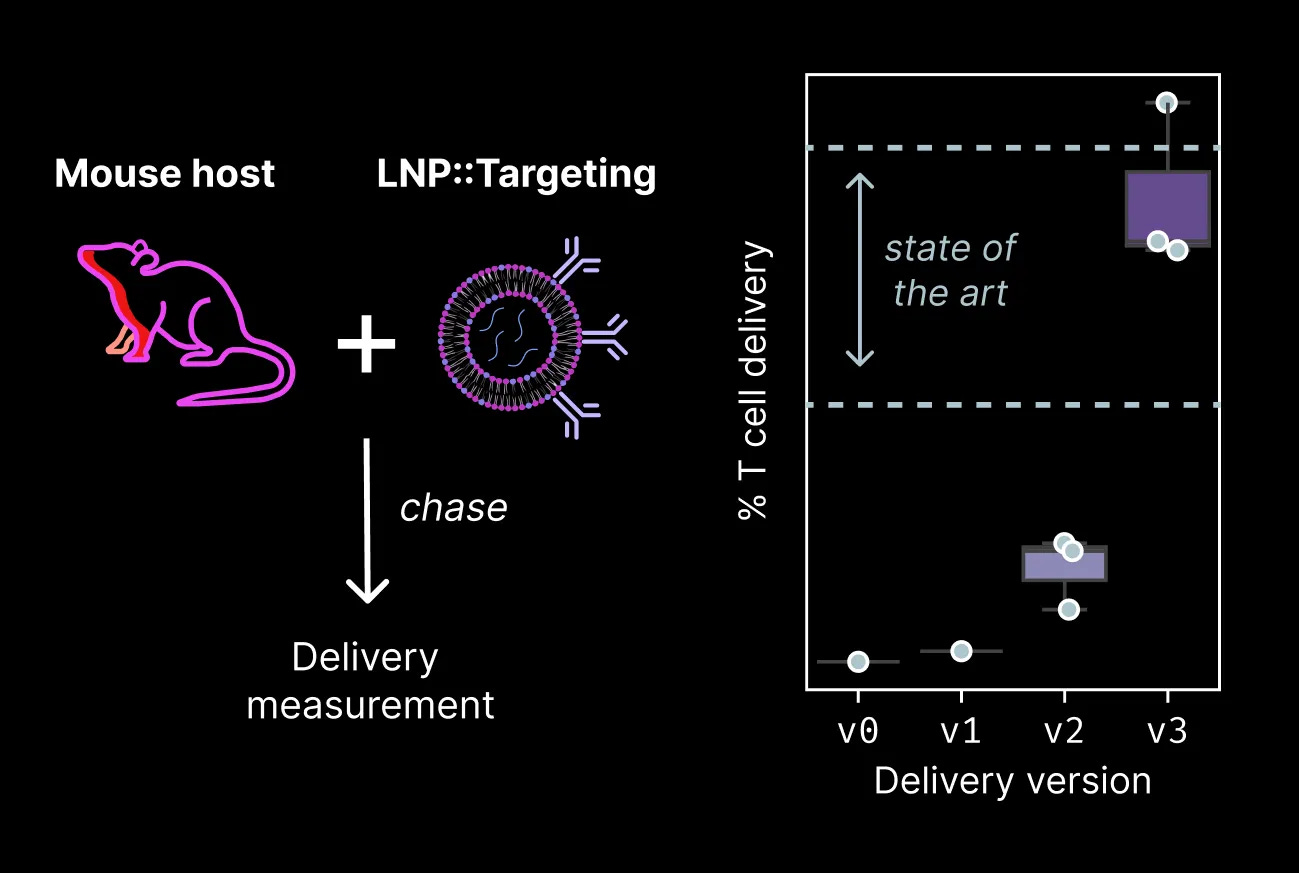
With these tools in hand, we can now test whether the payloads emerging from our Discovery Engine are effective in the complex environment of an organism.
Measuring endothelial cell age
Our most recent therapeutic program is focused on restoring function in endothelial cells of the vasculature. Before we can discover payloads that make old endothelial cells act young, we have to develop experimental assays to measure impairments with age.
Endothelial cell aging is dramatic enough that we’ve been able to discover functional impairments in just our first few experiments. Old human endothelial cells appear to be less regenerative than young cells in a simple culture system. Older tissues tend to lose the vascular networks that carry nutrients and signals throughout the body in a process known as “rarefaction.” These tissue level impairments may be connected to a cellular level loss of function like we’ve measured.
We further tested the impact of age in an animal model of endothelial injury in the kidneys. We found that aged animals display far more damage than their young counterparts, suggesting the aged endothelium may be less resilient to damage as well.
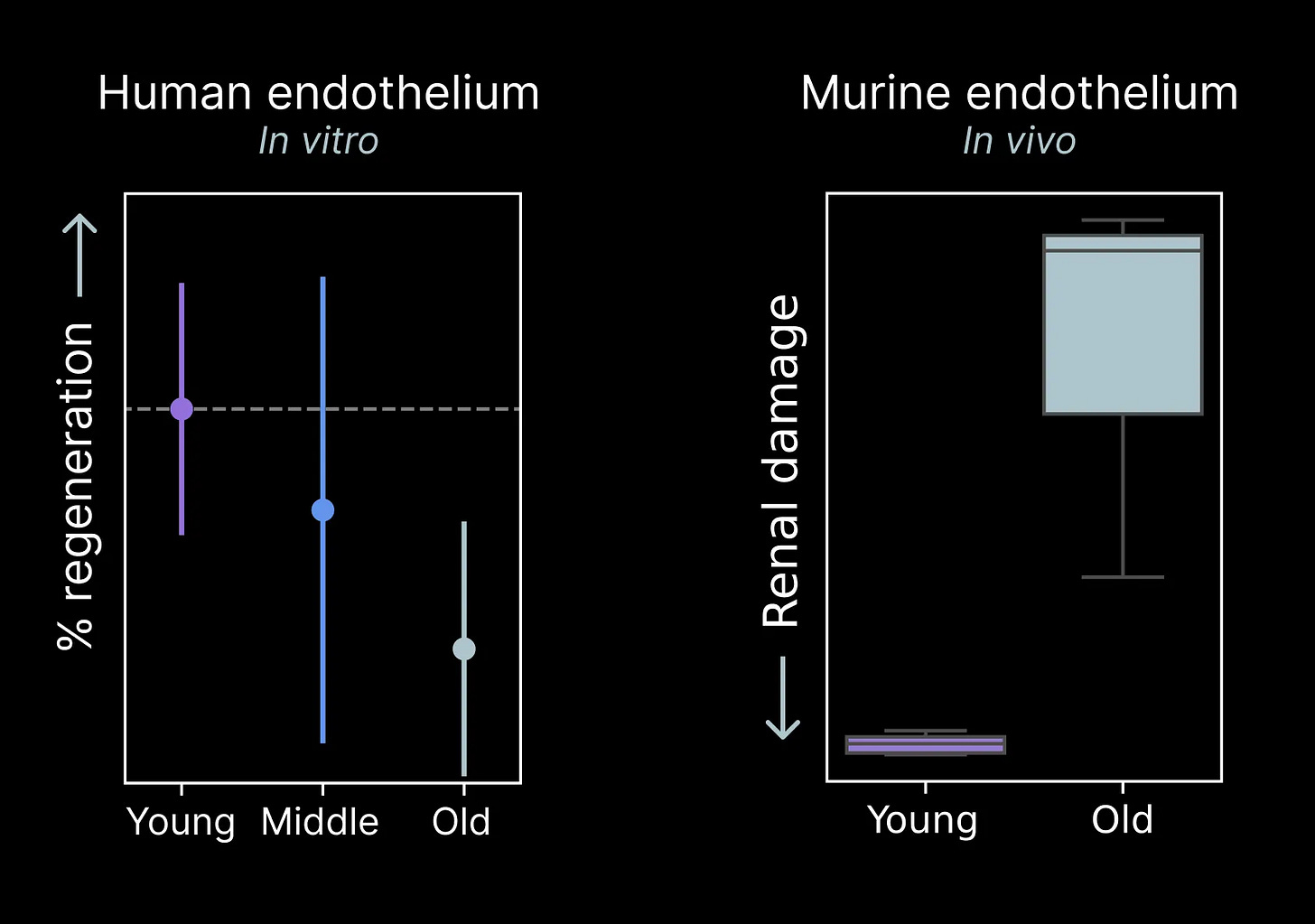
These are only the first of many functional evaluations we’ll employ. Already, they provide us with an initial tool to evaluate the functional effects of reprogramming payloads we discover.
Join our team
We’re recruiting for talented scientists across various departments. If you’re excited about the possibility of bringing the first reprogramming medicines to patients, please reach out.


